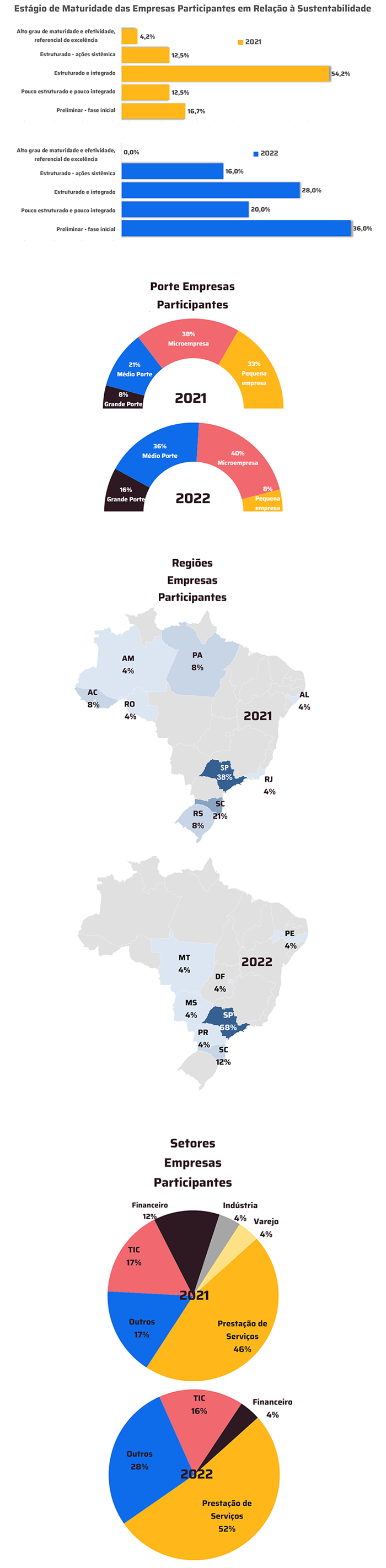
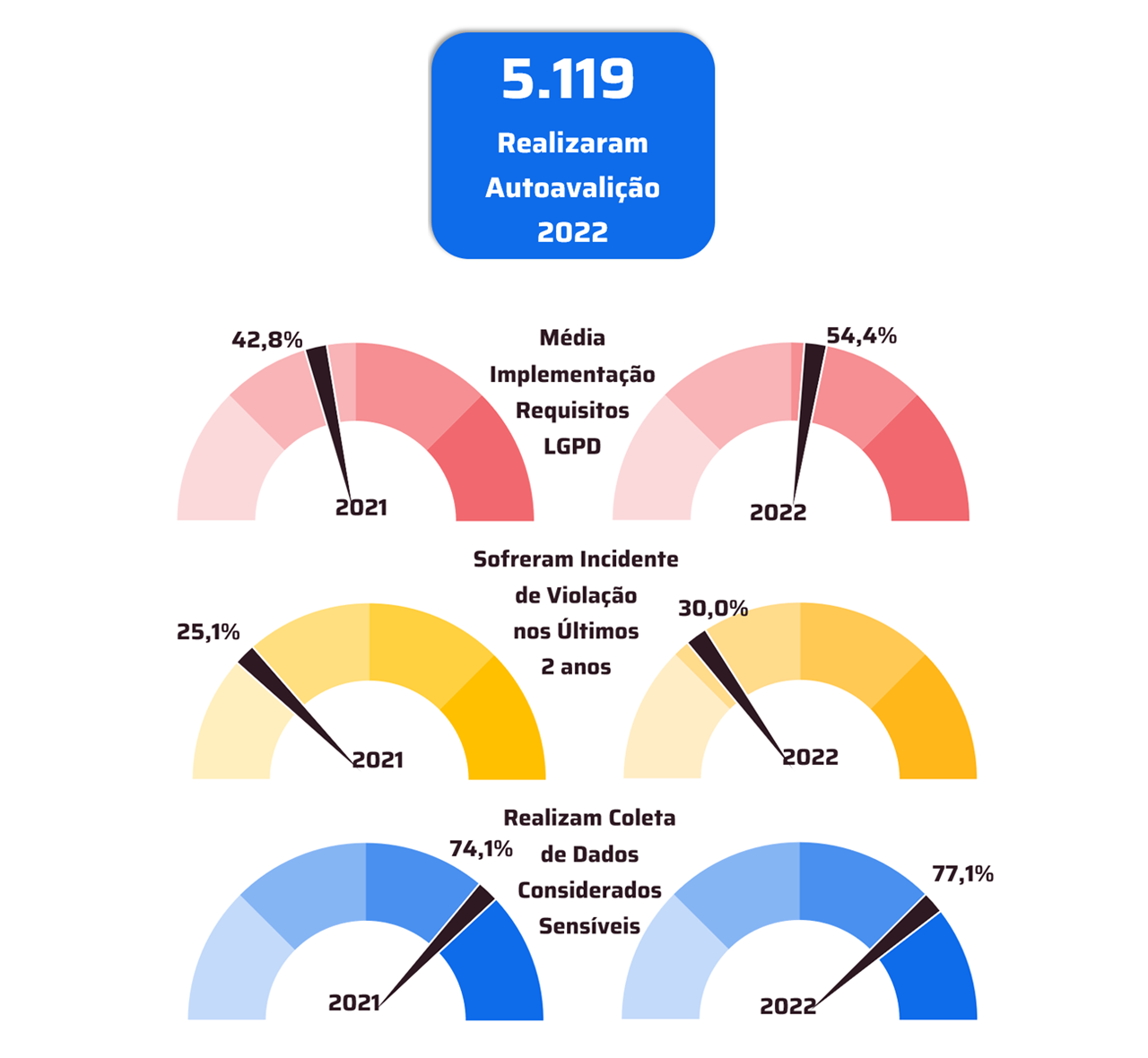
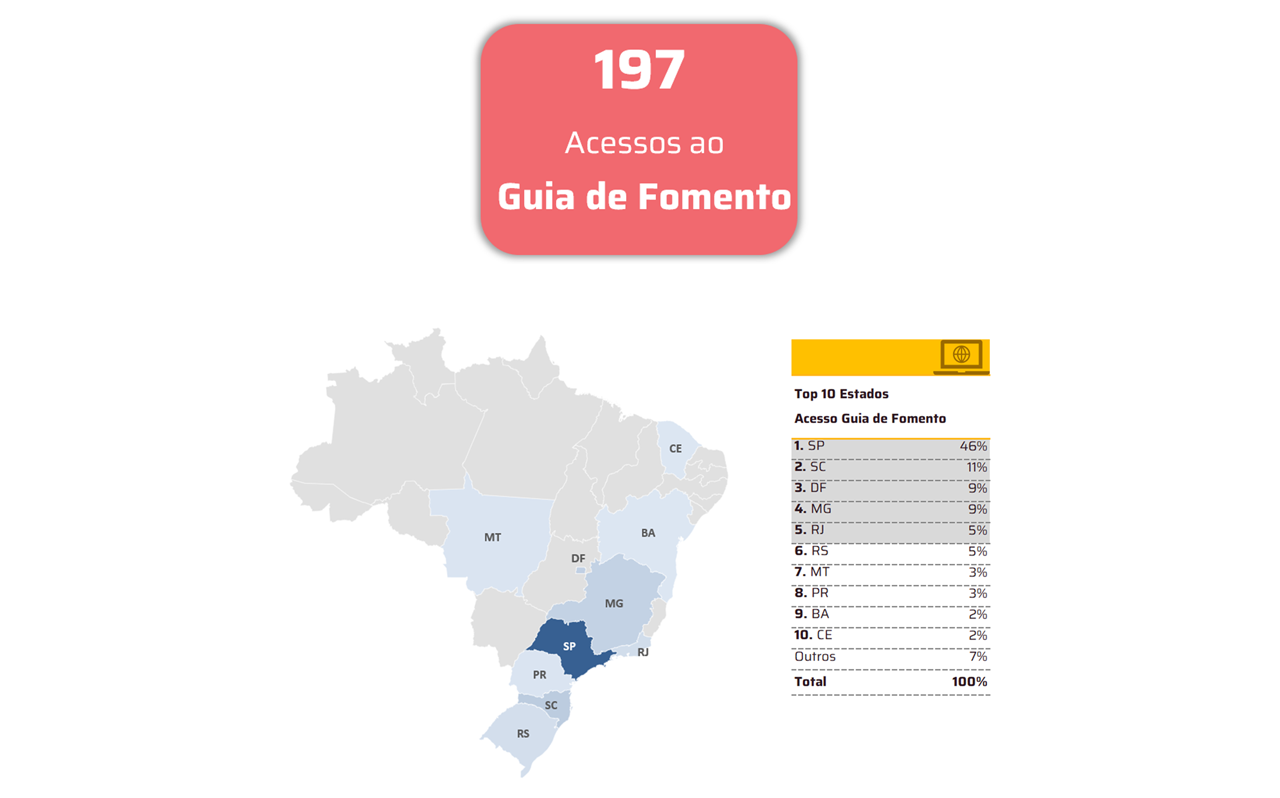
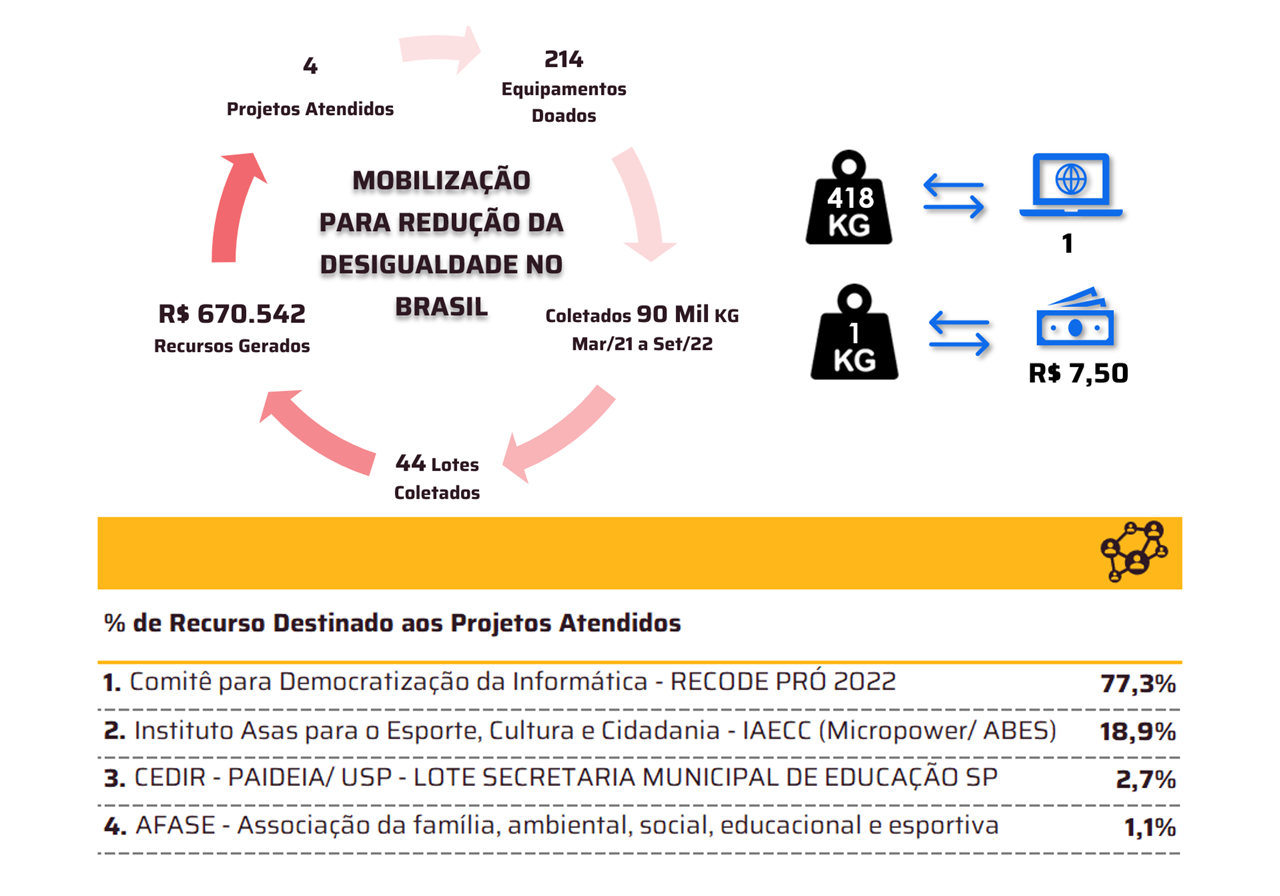
With TOTVS Backoffice – Protheus Line, the company has accurate information in its process to ensure compliance with the Anvisa standards required in the pharmaceutical market
The pandemic highlighted a series of challenges faced by the pharmaceutical industry, including the moment when drugs leave the manufacturer and need safe and efficient transport and storage until they reach their destination. Be it vaccines or thermolabile drugs, all require temperature and humidity control to ensure their effectiveness, in accordance with Anvisa's standards.

With over 20 years of experience, Grupo Polar is a specialist and pioneer in solutions to meet this Cold Chair (also known as Cold Chain) in the national market. In addition to manufacturing packaging for the transport of medicines, the Polar Group has the Valida Laboratory, which has a team of pharmacists and engineers who implement the qualification of refrigerated packaging, equipment, warehouses and fleets, in addition to refrigeration control at the time of transport. This level of qualification allows the company to operate in the vaccine segment, including those against COVID-19.
“Anvisa imposes a series of regulations on the monitoring of time and temperature of medications; our role is to provide, in addition to qualification, packaging and equipment that ensure the correct storage and transport of these medications within the established temperature range during all points in the chain – from the manufacturer to the administration to the patient. This becomes even more important with vaccines against COVID-19, since there are immunizations that require, for example, storage and thermal control at -70ºC, and given the current disease situation in Brazil, this is a process in which there cannot be errors that eventually result in wasted doses and their ineffectiveness in the population”, explains Gilberto Codarin, financial administrative director of Grupo Polar.
By working with inputs and products that require a lot of care and attention, Grupo Polar felt the need to hire a management system capable of centralizing information and helping to manage internal processes: “we lacked a consolidated base of information for decision making management and to operate the basic control management functions”, says the executive. To meet this demand, the Group chose TOTVS BackOffice – Protheus Line, which allowed the company to have better control of purchasing, receipts, inventory, accounts payable and production planning, ensuring productivity gains and information reliability.
“At the Valida Laboratory, we have seven air-conditioned chambers, which carry out customized temperature cycles and are constantly monitored. With the TOTVS system, we are able to work in an integrated manner, saving resources, increasing productivity and avoiding rework”, adds Codarin.
The Director of Manufacturing, Logistics and Agroindustry at TOTVS, Angela Gheller, reinforces the credibility and confidence that the choice of an ERP guarantees in a process as complex as the technologies involved in the cold chain. “Increasingly we observe and understand the importance of accurate data and information in daily operations, as well as in managerial and strategic decisions for the business. In the case of Grupo Polar, this need for real-time access to processes and information gains even more weight, since these are essential products”, says the executive.
“For us, the main advantage of the partnership with TOTVS was that we were able to work in an integrated manner, generating savings in resources, avoiding rework and greatly increasing our productivity”, concludes the director of Grupo Polar.